Mikel Uña-Gorospe1, Inmaculada Herrera-Mozo2, María Luïsa Canals3,
Gabriel Martí-Amengual1, Pere Sanz-Gallen1
1Unit of Occupational Medicine and Toxicology, School of Medicine and Health Sciences of Barcelona, University of Barcelona, Spain 2Unidad de Alergología, Clínica Creu Blanca, Barcelona, Spain
3Maritime Health Unit, ISM Tarragona, Spain
ABSTRACT
Background: Anisakis is a marine nematode. Its larvae can be found encysted in several species, both in the abdominal cavity and in the adjacent musculature. The most commonly affected commercial species are hake, whiting, cod, and mackerel. The prevalence in fish varies according to the fishing area and the size of the host.
Materials and methods: Until now only three species have been confirmed to be involved in human ani-sakiasis, the most common ones being A. simplex sensu stricto (s.s.) and A. pegreffii, and anecdotally, A. physeteris. Infestation in humans occurs when they eat raw or undercooked parasitised fish or cephalopods (pickled, cold-smoked, salted, semi-preserved, prepared in certain Asian styles like sushi or sashimi, ceviche). Results: The majority of anisakiasis cases have been described by Japanese authors. However, over the last few years there has been an increase in the number of cases reported in other countries including Italy and Spain. It is estimated that its incidence in the European Union is 0.32/100,000, and in the Basque Country (Spain), this parasite is responsible for 10% of anaphylaxis cases and 32% of urticaria cases in adults aged 40–60 years, around 300 cases/year. Anisakis-related disease in the work environment (oc-cupational disease) is less common.
Conclusions: We present three cases of the occupational disease in Spain due to a type I hypersensitivity to Anisakis simplex in individuals who handle fish (one fishmonger, one supermarket employee, and one chef).
INTRODUCTION
Anisakis is a marine nematode. Its larvae can be found encysted in several species, both in the abdominal cavity and in the adjacent musculature. Large marine mammals (ce-taceans and pinnipeds) are the natural definitive host, and the parasite matures to its adult stage in the gastrointestinal tract, from where its unembryonated eggs are released in the host’s faeces. The eggs then embryonate in the marine environment and the larvae mature in encapsulated phases (L1-2/L3), at a variable speed depending on the water tem-perature (4 to 8 days if between 13 and 18°C, 57 to 82 days if below 5°C) [1], until they begin the “free-swimming” L3 phase, in which they are still encapsulated, but can infect microcrustaceans, crustaceans, fish, and cephalopods. Co-pepods (microcrustaceans) and euphausiids (crustaceans — krill) act as intermediate hosts, and fish (teleosts) and cephalopods (squid in particular) are paratenic hosts, that is, while the larvae are transmitted through predation between fish and squid, they remain in L3, and if they are ingested by definitive hosts, they mature into L4 and the adult phase. If ingested by humans who have eaten infected fish, the larvae will only develop as far as stage L4 (Fig. 1) [2].
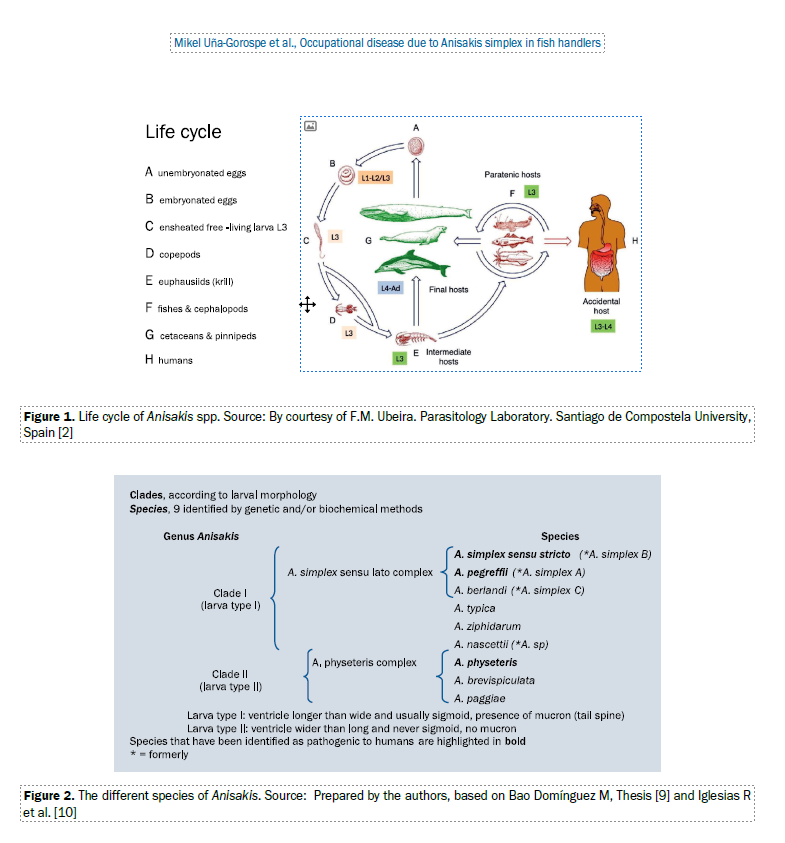
GEOGrAPHICAL DIStrIbUtION
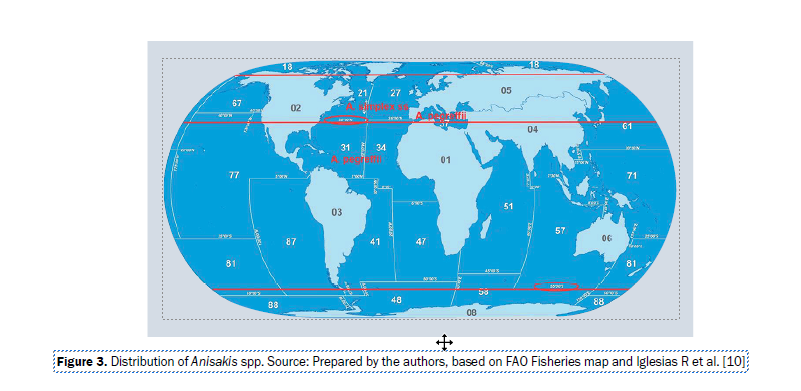
Anisakis are found from the Arctic Circle (66° 33’ 46” N) to 50° S, the species varying depending on the latitude. Anisakis simplex sensu stricto (s.s.) is found between the Arctic Circle and 35° N, except for the Mediterranean Sea, where the prevalent species is A. pegreffii, also found be-tween 35° N and 50° S latitude [3].
The most commonly affected commercial species are hake, whiting, cod, and mackerel, but the parasite has also been identified in a large variety of fish such as sole, john dory, sea bream, horse mackerel, herring, blue whiting, sardine, anchovy, haddock, monkfish, salmon, conger, and turbot, and in cephalopods, such as squid, octopus and cuttlefish [4–7]. We should also point out that studies confirm that the prevalence in fish varies according to the fishing area and the size of the host, with larger fish having more infestations [7, 8].
The different species identified are listed in Figure 2 [9, 10]; it is difficult or even impossible to differentiate the larval morphology based on microscopy alone.
PAtHOGENESIS
Until now only three species have been confirmed to be involved in human anisakiasis, the most common ones being A. simplex s.s. and A. pegreffii, and, anecdotally, A. physeteris. The geographic distribution of A. simplex s.s. and A. pegreffii, the species that are responsible for almost all cases of the disease described in humans, is presented in Figure 3 [10].
Infestation in humans occurs when they eat raw or un-dercooked parasitised fish or cephalopods, that is, those prepared with culinary techniques that do not kill the lar-vae. Thus, products that are pickled, cold-smoked, salted, semi-preserved, prepared in certain Asian styles (e.g. sushi, sashimi), or products such as ceviche, are responsible for practically all associated infections.
The first case of human infection by a species of the Ani-sakidae family was reported in the Netherlands by Van Thiel in 1960. The author described the presence of a marine nematode in the centre of an eosinophilic intestinal phleg-mon from a patient suffering from acute abdominal pain, as a “very unusual finding.” Later the nematode was identified as Anisakis spp., a common parasite of marine fishes and mammals, and the human parasitosis was named anisa-kiasis. Since then, the majority of anisakiasis cases have been described by Japanese authors, refiecting the frequent consumption of raw fish in that country [11].
However, over the last few years there has been an increase in the number of cases reported in other countries including Italy [8, 12] and Spain [6, 13].
The parasitic disease is caused by larvae in the gastro-intestinal tract following the ingestion of infected fish, in which the larvae are still alive and can hook onto the walls of the gastrointestinal tract. This parasitic infection may present in two ways:
— non-invasive or luminal form: the parasite attaches tothe stomach wall causing gastritis or even perforation, and in some cases migrating to the small intestine causing symptoms of enteritis, intestinal obstruction, or malabsorption syndrome;
invasive form: the larvae attach to and penetrate the intestinal mucosa and occasionally invade other organs (pancreas, liver, lungs) causing more severe symptoms.
The most common symptoms are those of intestinal inflammation: abdominal pain, nausea, vomiting, diarrhoea, and sometimes fever. Endoscopy is the most effective di-agnostic technique and can also be used therapeutically. It can also be helpful to perform total IgE serology testing, which is elevated in acute parasitic infection and decreases gradually, or Anisakis-specific IgE which increases in the first days of reinfection and remains high for months. Abdominal ultrasound or computed tomography can raise suspicion of the disease in cases of very active parasitic infection.
Involvement of the respiratory tract has also been de-scribed but is rare. One report describes “two patients that provide evidence for occupational asthma caused by A. simplex, based on in vivo and in vitro tests for Ani-sakis-specific IgE” [14].
Anisakiasis is a well-known, common, frequently-de-scribed disease. It is estimated that its incidence [15] in the European Union is 0.32/100 000, and in the Basque Country [16], this parasite is responsible for 10% of anaphylaxis cas-es and 32% of urticaria cases in adults aged 40–60 years, around 300 cases/year. Anisakis-related disease in the work environment (occupational disease) is less common.
We present three cases of occupational disease due to a type I hypersensitivity to Anisakis simplex in individuals who handle fish (one fishmonger, one supermarket employ-ee, and one chef).
CASE REPORTS
CASE 1
The first case was a 47-year-old woman, who had been working as a fishmonger for 27 years. She had a history of sensitivity to cobalt, nickel, and hair dyes, and had smoked 8 cigarettes per day for 25 years.
table 1. Complete battery of foods (Abelló and Allergy Therapeutics laboratories)*
*Controls: ALK Abelló laboratory: positive — histamine hydrochloride 10 mg/mL; negative — glycerinated saline
She reported a 1-year-history of pruritus and wheals, occurring predominantly on the upper limbs while at work, with remission of symptoms at weekends, during holidays, and while off work due to illness.
A skin prick test was performed with a complete battery of food allergens (Table 1), with negative results. Prick test (ALK Abelló laboratory) was negative to latex and positive to Anisakis simplex (12 × 15 mm papule; positive if ≥ 3 mm). She had a positive result of 11 × 12 mm to histamine hy- drochloride and a negative response to glycerinated saline. In vitro testing for specific IgE to Anisakis simplex (UniCAP; Pharmacia) was clearly positive with a result of
48 KU/L (positive if > 0.35 KU/L).
The diagnosis was compatible with recurrent acute ur- ticaria due to type I hypersensitivity to Anisakis simplex.
CASE 2
The second case was a 38-year-old woman with no relevant past personal or family medical diseases. She was a non-smoker, with no alcohol or drug abuse. She had been working in a supermarket for 10 years, specifically, in the delicatessen, butcher’s, and fish sections.
The patient reported that in the previous 6 weeks she had had two acute outbreaks of wheals with intense pru- ritus, on both occasions when she was working in the fish section.
In the first episode she had pruritic wheals on the hands and various parts of the body. Symptoms improved 6 hours after treatment with oral antihistamines and corticosteroids. Twelve days later, also while working in the fish section, she had a second episode of widespread wheals and intense pruritus. She was given 5 days of oral antihistamines and corticosteroids. The patient reported that she could not link the symptoms with ingestion of any foodstuff, and that she did not have any symptoms when working in the butcher or delicatessen sections. She was not taking any medications. A complete food skin prick test was performed (Table 1), which was negative. Prick test was also negative to latex and to additives used in the meat industry (Table 2). A prick by prick test for cereals used in minced meat was also negative.
table 2. Battery of additives used in the meat industry
Skin prick test was positive to Anisakis simplex (ALK Abelló laboratory), causing a 6 × 7 mm papule (positive if
≥ 3 mm); the response to histamine was 7 × 9 mm and to glycerinated saline, was negative.
In vitro tests detected a mildly positive response for
Anisakis simplex-specific IgE (UniCAP; Pharmacia) of
0.6 KU/L (positive if > 0.35 KU/L).
The diagnosis was compatible with acute urticaria due to type I hypersensitivity to Anisakis simplex.
CASE 3
The third case was a 37-year-old man, who had been working as a chef in a restaurant for 7 years. He had a history of seasonal rhinitis and had smoked 20 cigarettes per day for 18 years.
In the past 6 months he had had pruritic microvesicular le- sions on both hands with interphalangeal and palmar fissures. He reported that at work he handled fish, cephalopods (mainly cuttlefish) and crustaceans (prawns), and that his
symptoms improved when he was not working.
Skin prick tests with the complete battery of foods (Table 1) were performed, with negative results. Prick test to latex was also negative.
Prick test to Anisakis simplex (ALK Abelló laboratory) was positive with a 7 × 8 mm papule (positive if > 3 mm), a 9 ×
10 mm response to histamine, and a negative response to glycerinated saline.
In vitro testing gave the following results: elevated total IgE of 279 UI/L (high if > 150 UI/L). The results obtained from quantification of specific IgE to Anisakis simplex (Uni- CAP; Pharmacia) were positive at 0.8 KU/L (positive if > 0.35 KU/L).
The diagnosis was compatible with protein contact derma- titis due to type I hypersensitivity to Anisakis simplex.
In six healthy control patients a prick test was performed with the complete battery of foods, meat additives, latex, and Anisakis simplex, all of which were negative.
DISCUSSION
The spectrum of allergic reactions to Anisakis simplex is wide and includes rhinitis, conjunctivitis, asthma, urticaria/ angioedema, allergic contact dermatitis, and anaphylactic shock [4, 13, 14, 17].
The condition may be caused through the consumption of infected fish, or from occupational contact. The sectors most affected by Anisakis-related diseases are fishermen/ women, fishmongers, and chefs: in eneral, anyone whose work involves catching, cleaning, handling, selling, or pre- paring contaminated fish [13, 18].
Purello-D’Ambrosio et al. 2000 [18] conducted a study on sensitisation in fishermen and fishmongers, of 28 indi- viduals studied, four had a positive prick test to Anisakis simplex.
The cases we have discussed in this paper are all cases of occupational diseases secondary to Anisakis simplex including two cases of acute urticaria and one case of protein dermatitis, all of which are attributable to type I hypersensitivity to Anisakis simplex. These findings are in line with the results obtained by Conde-Salazar et al., 2002 [19]; Barbuzza et al., 2009 [20], and Vicente
Pardo, 2016 [5].
The diagnosis of the cases was based on the occupation- al history, the clinical presentation, the allergen exposure pattern (at work vs. off work), and the additional tests includ- ing both in vivo skin prick tests and in vitro tests quantifying Anisakis simplex-specific IgE levels.
Recently-developed molecular testing methods may be used to identify cross-reactivity between the protein from Anisakis and other allergenic sources such as crus- taceans. When a patient is diagnosed with hypersensitivity to Anisakis, a molecular test determines the exact protein to which they are sensitised and may therefore be useful for predicting the potential development of reactions to allergens that show cross-reactivity [21, 22].
Prevention should encompass tasks undertaken when fishing (gutting is performed on board the fishing boat), and in the subsequent handling of the product prior to (wholesalers)
and during (retailers) its sale to the final consumer, and, lastly, during food preparation. Thus, rapid gutting, correct freezing (see Food and Drug Administration [FDA] recommendations) and adequate cooking of fish are the most pertinent steps to avoid parasitic infection in the end consumer.
Regarding the prevention and treatment of the occupa- tional disease we have looked at, the best options to reduce exposure are barrier methods that protect the mucous membranes and exposed skin in individuals who carry out the tasks mentioned above, along with the provision of prior information and training.
The FDA recommendations [23] for parasite destruction by freezing (3-402.11) are:
- Except as specified in (B) of this section, before service or sale in READY-TO-EAT form, raw, raw-marinated, partially cooked, or marinated-partially cooked FISH shall be:
- Frozen and stored at a temperature of –20°C (–4°F) or below for a minimum of 168 hours (7 days) in a freezer;
- Frozen at –35°C (–31°F) or below until solid and stored at –35°C (–31°F) or below for a minimum of 15 hours; or
- Frozen at –35°C (–31°F) or below until solid and stored at –20°C (–4°F) or below for a minimum of 24 hours.
- Paragraph (A) of this section does not apply to:
- MOLLUSCAN SHELLFISH;
- A scallop product consisting only of the shucked adductor muscle;
- Tuna of the species Thunnus alalunga, Thunnus alb- acares (Yellowfin tuna), Thunnus atlanticus, Thunnus maccoyii (Bluefin tuna, Southern), Thunnus obesus (Bigeye tuna), or Thunnus thynnus (Bluefin tuna,
Northern); or
- Aquacultured FISH, such as salmon, that:
- If raised in open water, are raised in net-pens, or
- Are raised in land-based operations such as ponds or tanks, and
- Are fed formulated feed, such as pellets, that contains no live parasites infective to the aqua- cultured FISH;
- FISH eggs that have been removed from the skein and rinsed.
CONCLUSIONS
Health conditions caused by Anisakis are highly relevant for consumers of fish and represent a significant public health issue. It should also be borne in mind that exposure to Anisakis can cause an occupational disease in individuals who handle fish (particularly fishermen/women, fishmon- gers, supermarket workers, and chefs).
Preventative measures should be strengthened to avoid or minimise the health effects that exposure to Anisakis can cause, both in the general population and in those exposed in their work environment.
REFERENCES
- Audicana M, Pozo Md, Iglesias R, et al. Anisakis simplex and Pseu- doterranova decipiens. In: Miliotis MD and Bier JW. International Handbook of Foodborne Pathogens, Marcel Dekker Inc; New York. 2003; Ch.l 38(pp:615).
- Iglesias R, Martínez V y Ubeira FM “Aspectos básicos de la An- isakiosis” [Internet], figure 1 ln pp 8. Conference paper; 2015. https://www.researchgate.net/profile/Florencio_Ubeira/publica- tion/284723919_Aspectos_basicos_que_deberiamos_saber_so- bre_Anisakis/links/5a4a8e26458515f6b05b3877/Aspectos-ba- sicos-que-deberiamos-saber-sobre-Anisakis.pdf (cited 2018 Nov 24).
- Audicana MT, Kennedy MW. Anisakis simplex: from obscure infec- tious worm to inducer of immune hypersensitivity. Clin Microbiol Rev. 2008; 21(2): 360–79, table of contents, doi: 10.1128/CMR.00012-07, indexed in Pubmed: 18400801.
- Nieuwenhuizen N, Lopata AL, Jeebhay MF, et al. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. J Allergy Clin Immunol. 2006; 117(5): 1098–1105, doi: 10.1016/j.jaci.2005.12.1357, indexed in Pubmed: 16675338.
- Vicente Pardo JM. El anisakis como enfermedad profesional. Med Segur Trab. 2016; 62(244): 223–240.
- Molina-Fernández D, Malagón D, Gómez-Mateos M, et al. Fishing area and fish size as risk factors of Anisakis infection in sardines (Sardina pilchardus) from Iberian waters, southwestern Europe. Int J Food Microbiol. 2015; 203: 27–34, doi: 10.1016/j.ijfoodmi- cro.2015.02.024, indexed in Pubmed: 25770431.
- Bušelić I, Botić A, Hrabar J, et al. Geographic and host size variations as indicators of Anisakis pegreffii infection in European pilchard (Sardina pilchardus) from the Mediterranean Sea: Food safety impli- cations. Int J Food Microbiol. 2018; 266: 126–132, doi: 10.1016/j. ijfoodmicro.2017.11.021, indexed in Pubmed: 29216552.
- Guardone L, Nucera D, Lodola LB, et al. Anisakis spp. larvae in dif- ferent kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. Int J Food Microbiol. 2018; 268: 10–18, doi: 10.1016/j.ijfoodmicro.2017.12.030, indexed in Pubmed: 29306733.
- Bao Dominguez M. A multidisciplinary approach to tackle the problem of the zoonotic parasite Anisakis in fish [nternet], Chapter 1-General intro- duction, 1-2.The genus Anisakis, pg 17-19; thesis presented for the De- gree of Doctor of Philosophy in Systems Biology at the University of Aber- deen, April 2017. http://digital.csic.es/bitstream/10261/152258/1/ Tesis_Miguel_Bao.pdf (cited 2018 Nov 24).
- Iglesias R, Martínez V y Ubeira FM “Aspectos básicos de la Anisaki- osis” [nternet] pg1. Conference paper 2015.https://www.research- gate.net/profile/Florencio_Ubeira/publication/284723919_Aspectos_basicos_que_deberiamos_saber_sobre_Anisakis/ links/5a4a8e26458515f6b05b3877/Aspectos-basicos-que-debe- riamos-saber-sobre-Anisakis.pdf (cited 2018 Nov 24).
- Audicana M, Pozo Md, Iglesias R, et al. Anisakis simplex and Pseu- doterranova decipiens. In: Miliotis MD and Bier JW. International Handbook of Foodborne Pathogens, Marcel Dekker Inc; New York. 2003; Ch.l 38(pp:613).
- Heffler E, Sberna ME, Sichili S, et al. High prevalence of Anisakis sim- plex hypersensitivity and allergy in Sicily, Italy. Ann Allergy Asthma Im- munol. 2016; 116(2): 146–150, doi: 10.1016/j.anai.2015.12.014, indexed in Pubmed: 26815707.
- Ivanović J, Baltić M, Bošković M, et al. Anisakis allergy in hu- man. Trends in Food Science & Technology. 2017; 59: 25–29, doi:10.1016/j.tifs.2016.11.006.
- Armentia A, Lombardero M, Callejo A, et al. Occupational asthma by Anisakis simplex. J Allergy Clin Immunol. 1998; 102(5): 831–834, indexed in Pubmed: 9819301.
- Orphanet Report Series – Prevalence of rare diseases: Bibliographic data [nternet] June 2018 – Number 1.http://www.orpha.net/orpha- com/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabet- ical_list.pdf (cited 2018 Nov 24).
- Elika, Fundación Vasca para la Seguridad Agroalimentaria [Internet] website April 2010 . http://ciudadania.elika.eus/alergia_alimentar- ia_anisakis.asp(updated 2018 Aug 1, cited 2018 Nov 24).
- Daschner A, Pascual CY. Anisakis simplex: sensitization and clini- cal allergy. Curr Opin Allergy Clin Immunol. 2005; 5(3): 281–285, doi:10.1097/01.all.0000168795.12701.fd.
- Purello-D’Ambrosio F, Pastorello E, Gangemi S, et al. Incidence of sensitivity to Anisakis simplex in a risk population of fisher- men/fishmongers. Ann Allergy Asthma Immunol. 2000; 84(4): 439–444, doi: 10.1016/S1081-1206(10)62278-8, indexed in
Pubmed: 10795653.
- Conde-Salazar L, González MA, Guimaraens D. Type I and Type IV sen- sitization to Anisakis simplex in 2 patients with hand eczema. Con- tact Dermatitis. 2002; 46(6): 361, indexed in Pubmed: 12190630.
- Barbuzza O, Guarneri F, Galtieri G, et al. Protein contact dermatitis and allergic asthma caused by Anisakis simplex. Contact Dermatitis. 2009; 60(4): 239–240, doi: 10.1111/j.1600-0536.2009.01519.x,
indexed in Pubmed: 19338604.
- Moneo I, Carballeda-Sangiao N, González-Mu oz M. New Perspec- tives on the Diagnosis of Allergy to Anisakis spp. Curr Allergy Asthma Rep. 2017; 17(5): 27, doi: 10.1007/s11882-017-0698-x, indexed
in Pubmed: 28429304.
- Armentia A, Santos J, Serrano Z, et al. Molecular diagnosis of allergy to Anisakis simplex and Gymnorhynchus gigas fish parasites. Aller- gol Immunopathol (Madr). 2017; 45(5): 463–472, doi: 10.1016/j. aller.2016.12.008, indexed in Pubmed: 28341528.
- Food Code 2017. Recommendations of the United States Public Health Service [Internet] Food and Drug Administration, pp: 89-90. https://www.fda.gov/Food/GuidanceRegulation/RetailFoodProtec- tion/FoodCode/ucm595139.htm (cited Nov 24).


