1 Clinica Creu Blanca, Barcelona, Spain Unit of Allergy
2 University of Barcelona, Barcelona, Spain
Unit of Occupational Medicine, School of Medicine
Abstract
Omeprazole is a proton pump inhibition and ranitidine is an H2 histamine receptor antagonist widely used in the treatment of gastroesophageal reflex disease, peptic ulcer disease, Zollinger-Ellison syndrome and as a protector of the gastric mucosae. We re- port a case of occupational contact allergy to omeprazole and ranitidine. A 48-year-old man, with no pre-existing history of atopy or lifestyle factors. He neither had any medical history of consumption of drugs such as ranitidine and omeprazole. He worked for 19 months in the pharmaceutical company that manufactured ranitidine base. He presented rash in the face and eczema on the dorsum of the hands with itching. The study by prick tests with ranitidine gave negative response. Patch testing with ranitidine base and ranitidine hydrochloride gave positive response. A month later, when the patient was asymptomatic he returned to the pharmaceutical company, being switched from this previous job to the reactor manufacturing omeprazole. A few days after that, he presented erythematous eruptions involving face and neck with itching. Prick tests, path tests and in vitro laboratories stud- ies with omeprazole gave positives. In this case the patient presented hypersensitivity type I at omeprazole and hypersensitivity type IV at omeprazole and ranitidine. Our aportation indicates the importance of careful analysis of the occupational exposure histories of patients with the suspected type I or type IV hypersensitivity to allergens, to determine whether work exposure is the cause. Med Pr 2017;68(3):433–435
Key words: occupational exposure, occupational contact dermatitis, proton pump inhibitors, ranitidine, omeprazole, active pharmaceutical ingredients
Corresponding author: Pere Sanz-Gallen, University of Barcelona, Unit of Occupational Medicine, School of Medicine, Casanova 143, 08036 Barcelona, Spain, e-mail: 17039psg@comb.cat
Received: June 2, 2016, accepted: December 4, 2016
INTRODUCTION
Occupational exposure to active pharmaceutical in- gredients may cause adverse health effects [1]. Althou- gh omeprazole is potentially sensitizing, occupatio- nally acquired cases in pharmaceutical industry are rare [2–6].
We report one case of occupational contact allergy to omeprazole and ranitidine.
CASE REPORT
A 48-year-old man, with no pre-existing history of atopy or lifestyle risk factors. Neither did he have any medical history of consumption of drugs such as ra- nitidine and omeprazole. He worked for 19 months in the synthesis of active ingredients for a pharmaceutical company that manufactured ranitidine base. He pre- sented rash in the face and eczema on the dorsum of the hands with itching. Skin symptoms were triggered in the first 4–6 h of starting the workday, and required
treatment with corticosteroid topical and anti-histami- ne oral for resolution. The patient did not develop skin reactions in periods without work activity (weekends and holiday periods).
Prick tests with ranitidine were performed at 30 mg/ ml and 3 mg/ml, and gave negative response.
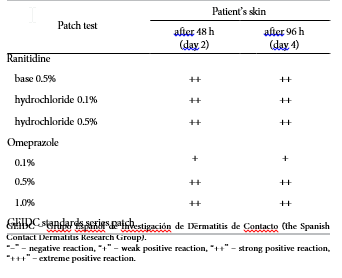
Patch testing was done according to the GEIDC (Grupo Español de Investigación de Dermatitis de Contacto – the Spanish Contact Dermatitis Research Group) standard series and patch testing – with rani- tidine base (5% pet), ranitidine hydrochloride (1% pet) and ranitidine hydrochloride (5% pet). Results were read at 48 h (day 2) and 96 h (day 4), which are descri- bed in the Table 1. Prick tests and path tests gave nega- tive results in all 10 healthy controls.
A month later, when the patient was asymptoma- tic, he returned to the pharmaceutical company, being switched from his previous job to the reactor manufac- turing omeprazole. A few days after that, he presented erythematous eruptions involving face and neck with itching.
Table 1. Patch test of the 48-year-old patient with contact allergy to omeprazole and ranitidine
In this case the patient presented hypersensitivity type I at omeprazole and hypersensitivity type IV at omeprazole and ranitidine. Both omeprazole and rani- tidine are low molecular weight substances (354.42 Da and 350.9 Da, respectively), and may therefore trigger allergic pathology mediated by a type I hypersensitivi- ty mechanism. This patient developed type IV hyper- sensitivity against both omeprazole and ranitidine, but also type I against omeprazole, which has supported the relevance of low molecular weight allergens in the de- velopment of allergic disease by different mechanisms.
GEIDC – Grupo Español de Investigación de Dermatitis de Contacto (the Spanish Contact Dermatitis Research Group).
“–” – negative reaction, “+” – weak positive reaction, “++” – strong positive reaction, “+++” – extreme positive reaction.
Prick tests with omeprazole were performed at concentration of 20 mg/ml and the time of reading was 12 min, obtaining a positive result: papule 6×8 mm, suggesting immediate-type allergy.
In vitro laboratory studies, consisting of a histamine
release test by radioimmunoassay, were also performed to support the results obtained through prick testing: this test showed positive results with omeprazole, with a percentage rate of histamine release of 42% (cut-off value in the test: 30%).
Omeprazole was patch-tested in saline solution at 0.1%, 0.5% and 1%. Results were read at 48 h (day 2) and 96 h (day 4), and are described in the Table 1. Prick tests and patch tests gave negative results in 10 healthy controls.
DISCUSSION
Omeprazole is a proton pump inhibitor. It is admini- stered orally or intravenously, and is widely used in the treatment of gastroesophageal reflex disease, peptic ul- cer disease, and Zollinger-Ellison syndrome.
Ghatan et al. (2014) [7] provides a study of 96 wor- kers exposed to omeprazole during manufacturing pro- cess for suspected allergy. Twenty-eight workers have positive patch tests. The results of prick tests were all ne- gative. Confino-Cohen and Golberg (2006) [8] proposed a desensitization protocol for omeprazole anaphylaxis.
Ranitidine is an H2 histamine receptor antagonist, used in the treatment of duodenal ulcers and gastric
CONCLUSIONS
We cannot conclude whether the sensitization to rani- tidine and omeprazole in the case of this patient is due to a cross-sensitivity or a co-sensitivity, since no in vitro studies have been carried out by immunoblotting.
Our aportation indicates the importance of careful analysis of the occupational exposure histories of pa- tients with suspected type I or type IV hypersensitivity to allergens, in order to determine whether work expo- sure is the actual cause.
REFERENCES
- Heron RJ, Pickering C. Health effects of exposure to active pharmaceutical ingredients (APIs). Occup Med (Lond). 2003;53(6):357–62, https://doi.org/10.1093/occ- med/kqg115.
- Meding B. Contact allergy to omeprazole. Contact Der- matitis. 1986;15(1):36, https://doi.org/10.1111/j.1600-05 36.1986.tb01259.x.
- Conde-Salazar L, Blancas Espinosa R, Pérez Hortet C. Oc- cupational airborne contact dermatitis omeprazole. Contact Dermatitis. 2007;56(1):44–6, https://doi.org/10.1111/j.1600- 0536.2007.00921.x.
- Sanz-Gallen P, Nogué S, Herrera-Mozo I, Delclos G, Vale- ro A. Occupational contact allergy to omeprazole and flu- oxetine. Contact Dermatitis. 2011;65(2):118–9, https://doi. org/10.1111/j.1600-0536.2011.01931.x.
- Alarcon M, Herrera-Mozo I, Nogué S, Sanz-Gallen P. Oc- cupational airborne contact dermatitis from proton pump inhibitors. Curr Allergy Clin Immunol. 2014;27(4):310–3.
- Yu AM, DeKoven JG. Occupational airborne contact der- matitis from proton pump inhibitors. Dermatitis. 2015;
Nr 3 Contact allergy to omeprazole and ranitidine 435
26(6):287–90, https://doi.org/10.1097/DER.0000000000000139.
- Ghatan PH, Marcusson-Stahl M, Matura M, Björkhe- den C, Lundborg P, Cederbrant K. Sensitization to ome- prazole in the occupational setting. Contact Dermatitis. 2014(6);71:371–5, https://doi.org/10.1111/cod.12305.
- Confino-Cohen R, Golberg A. Anaphylaxis to omeprazole diagnosis and desensitization protocol. Ann Allergy Asth- ma Immunol. 2006;96(1):33–6, https://doi.org/10.1016/ S1081-1206(10)61037-X.
- Romaguera C, Grimalt F, Vilaplana J. Epidemic of occu- pational contact dermatitis from ranitidine. Contact Der- matitis. 1988;18(3):177–8, https://doi.org/10.1111/j.1600- 0536.1988.tb04512.x.
- Ryan PJ, Rycroft RJ, Aston IR. Allergic contact dermatitis from occupational exposure to ranitidine hydrochloride. Contact Dermatitis. 2003;48(2):67–8, https://doi.org/10. 1034/j.1600-0536.2003.480202.x.


