María Alarcón, MD1
Inmaculada Herrera-Mozo, MD2
Santiago Nogué, MD, PhD1
Pere Sanz-Gallen, MD, PhD1,3
1. Clinical Toxicology Unit, Hospital Clinic of Barcelona, Spain
2. Unit of Allergy, Cínica Creu Blanca. Barcelona, Spain
3. School of Occupational Medicine, University of Barcelona, Spain
Correspondence:
Pere Sanz-Gallen, email: 17039psg@comb.cat
Keywords: Contact Dermatitis; Proton Pump Inhibitors; Lansoprazole; Omeprazole; Pantoprazole; Active Pharmaceutical Ingredients
ABSTRACT
Proton Pump Inhibitors (PPI) are members of the benzimidazole family, and are commonly used in the treatment of acid-related disorders such as gastroesophageal reflux disease, peptic ulcer disease, associated Helicobacter pylori infection and Zollinger-Ellison syndrome, as they are potent inhibitors of gastric acid secretion. We report three cases of occupational airborne contact dermatitis from proton pump inhibitors (lansoprazole, omeprazole and pantoprazole) among workers in the pharmaceutical industry.
INTRODUCTION
Occupational exposure to active pharmaceutical ingredients can cause adverse health effects.1 Depending on the type of product and exposure, several occupational dermatoses have been described among workers in the pharmaceutical industry, including irritation, contact allergy, photosensitivity, urticaria, acne venerate, and less frequently, fixed drug eruptions, steroid-induced rosacea, and even toxic epidermal necrolysis.2
Contact dermatitis from exposure to Proton Pump Inhibitors (PPI), such as lansoprazole,3 omeprazole4,5,6 and pantoprazole7 are quite uncommon. We report three cases of occupational airborne contact dermatitis from PPI.
CASE REPORTS
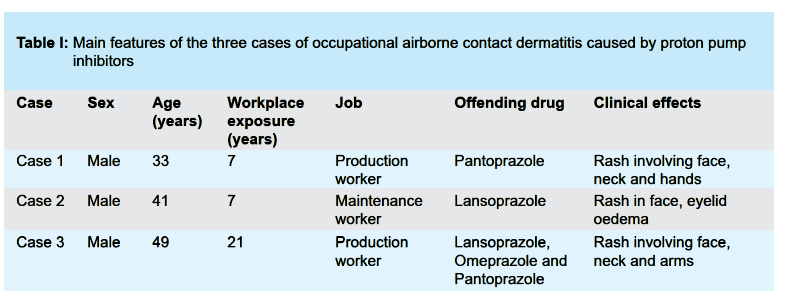
The three cases correspond to male workers in the pharmaceutical industry between the ages of 33 and 49 and an exposure time ranging from 7 to 21 years. The time between the onset of clinical manifestations and diagnosis ranged was between 1 and 9 months. None of the workers had a prior history of atopy. Two were production workers and the other a maintenance worker. The clinical signs and symptoms would appear when they were in contact with PPI, and would decrease or disappear when not exposed to PPI.
Clinical manifestations in the production workers consisted of rashes involving the face, neck, and upper extremities. The maintenance worker presented with a facial rash and swelling of the eyelids.
All three cases responded to symptomatic treatment with oral corticosteroids. The main clinical features of the three patients are described in Table I (see next page), including sex, age, duration of exposure prior to clinical manifestation, work activity, offending drugs and clinical effects.
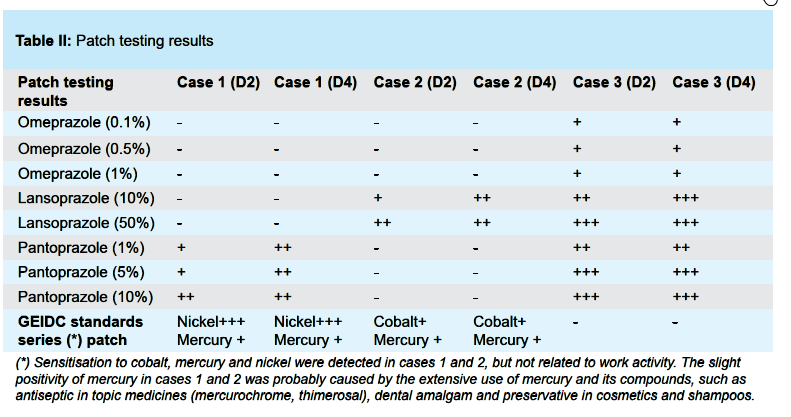
Both skin prick and patch testing were performed. Prick tests were performed at sub-irritant concentrations: lansoprazole (15 mg/1 ml of saline solution), omeprazole (40 mg/ml) and pantoprazole (20 mg/ml). Patch testing was done with Grupo Español Investigación Dermatitis de Contacto (GEIDC) standard series: lansoprazole (10%, 50%), omeprazole (0.1 %, 0.5 %, 1 %) and pantoprazole (1%, 5 %, 10 %), all in saline solution. Results were read at 48 hours (D2) and 96 hours (D4) are described in Table II (see next page).
Prick tests and patch tests gave negative results in all 10 healthy controls. In all three cases, the prick test results were negative. Patch testing results were positive in one case for lansoprazole, other case for pantoprazole, the last case for lansoprazole, omeprazole and pantoprazole. They are described in Table II.
DISCUSSION
PPI are members of the benzimidazole family, and are commonly used in the treatment of acid-related disorders such as gastroesophageal reflux disease, peptic ulcer disease, associated Helicobacter pylori infection and Zollinger-Ellison syndrome, as they are potent inhibitors of gastric acid secretion.8
There are very few cases described of airborne occupational contact dermatitis from occupational exposure to PPI. In the case of omeprazole, Meding, 1986,4 reported the first two
cases, Conde-Salazar et al, 20075 added two others, and Sanz-Gallen et al 2011 provided a fifth new case.6 Only one case each has been described in association with exposure to lansoprazole3 and pantoprazole.7
The addition of these three cases, one for each of these commonly used PPI, is important because it underscores their sensitising potential, which should be considered when describing the adverse effects of active pharmaceutical ingredients.9
ACKNOWLEDGEMENTS
We would like to thank Professor George Delclos (School of Public Health, University of Texas, Houston, USA and Centre for Research in Occupational Health, Pompeu Fabra University, Barcelona, Spain) for his comments on a previous version of this article.
REFERENCES
- Heron RJL, Pickering FC. Health effects of exposure to active pharmaceutical ingredients (APIs). Occup Med (Lond) 2003;53:357-362.
- Goossens AN, Van der Hulst K. Occupational contact dermatitis in the pharmaceutical industry. Clinics in Dermatology 2011;29:662-668.
- Vilaplana J, Romaguera C. Allergic contact dermatitis due to lansoprazole, a proton pump inhibitor. Contact Dermatitis 2001;44:47-48.
- Meding B. Contact allergy to omeprazole. Contact Dermatitis 1986;15:36.
- Conde-Salazar L, Blancas-Espinosa R, Pérez-Hortet C. Occupational airborne contact dermatitis from omeprazole. Contact Dermatitis 2007;56:44-46.
- Sanz-Gallen P, Nogué S, Herrera-Mozo I, Delclos GL, Valero A. Occupational contact allergy to omeprazole and fluoxetine. Contact Dermatitis 2011;65:118-119.
- Neumark M, Ingber A, Levin M, Slodownik D. Occupational airborne contact dermatitis caused by panteprazole. Contact Dermatitis 2010;64:60-61.
- Chang Y-S. Hypersensitivity reactions to proton pumps inhibitors. Curr Opin Allergy Clin Immunol 2012;12:348-353.
- Binks SP. Occupational toxicology and the control of exposure to pharmaceutical agents at work. Occup Med (Lond) 2003;53:363-370.


